
Pancreatic cancer has garnered a daunting reputation, often referred to as a “silent killer” due to its notoriously covert and ambiguous symptomatology. Pancreatic cancer is a leading cause of cancer-related deaths globally, with about 80% of patients with pancreatic cancer diagnosed with locally advanced or distant metastatic disease when long-term survival is extremely uncommon. Notables like Steve Jobs, actor Patrick Swayze, and opera legend Luciano Pavarotti were in the public eye but silently battled this deadly disease, eventually succumbing to its grip, underscoring an alarming global concern.
The majority of pancreatic cancer cases are currently found when they are in stage III or IV, and stage I detection would result in the best survival chances. The difference between the actual and optimal detection stages has a considerable negative impact on post-diagnosis quality of life and survival rates.
AI-driven solution
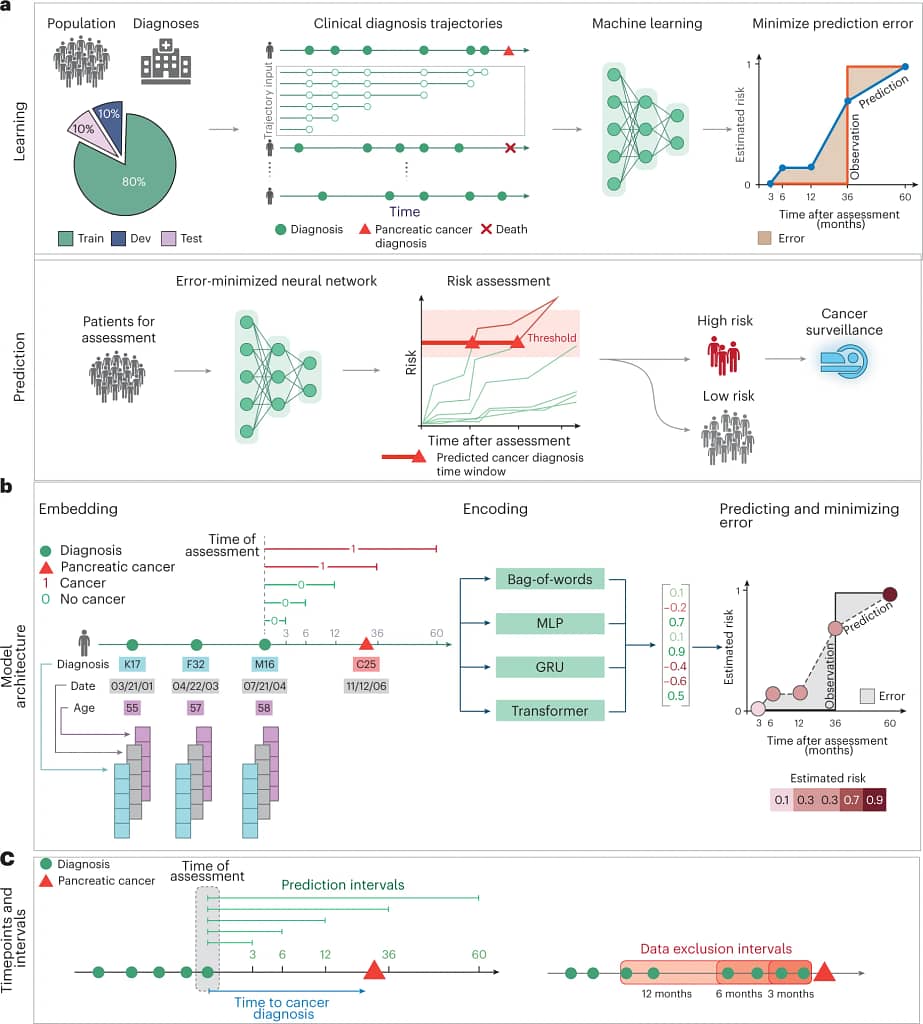
The AI solution for pancreatic cancer detection uses advanced machine learning and combines Transformer models (scalable deep learning models capable of focusing on critical data through attention mechanisms) and Gated Recurrent Units (GRUs), a type of neural network skilled at managing sequential data by selectively remembering or forgetting past information. The models combine the power of GRUs in comprehending the temporal (time-related) progression of disease trajectories with the astuteness of Transformer models in identifying pertinent data patterns.
Type of input data leveraged
- The AI model relies on a wealth of patient data. It starts with the comprehensive disease diagnosis trajectories stored in medical records. These trajectories capture the historical journey of a patient’s health, using specific International Classification of Diseases (ICD) codes to represent various diagnoses. This means that every ailment, medical condition, or symptom documented over time is included in the analysis.
- Temporal data is another critical component. This information accounts for when each diagnosis occurred, providing a chronological view of a patient’s medical history. It allows the AI model to discern patterns and trends in disease progression over time, offering invaluable insights.
- Furthermore, age-related inputs play a significant role. Age has been linked to pancreatic cancer incidence, and incorporating this factor into the analysis strengthens the model’s predictive capabilities. By considering a patient’s age alongside their disease trajectory, the AI system gains a more holistic view of their health profile.
- Together, these data elements form a robust foundation for the AI model’s predictions, enabling it to assess pancreatic cancer risk with precision and accuracy, potentially revolutionizing early detection in healthcare.
Data-backed impact of solution
Employing this AI-driven approach has shown measurable, promising results in the early detection of pancreatic cancer. The model’s evaluation highlighted its capacity to reliably predict potential cancer risks; for instance, in predicting cancer occurrence within 36 months, the model achieved an impressive accuracy rate, as denoted by an Area Under the Receiver Operating Characteristic (AUROC) curve of 0.88. To break this down, the closer this value is to 1, the better the model is at distinguishing between patients with and without the disease. When focused on an even shorter time frame, excluding events just 3 months before a potential diagnosis, this accuracy is slightly adjusted to an AUROC of 0.83. In real terms, this means that for every 1,000 high-risk patients over the age of 50, there’s an estimated relative risk of 59.
This technology is not just an academic exercise; it has practical, real-world potential. Its high accuracy rates suggest it can be an invaluable tool in early cancer detection, significantly increasing the chances of timely intervention and successful treatment for patients.
Possible hindrances to implementation and solutions
The path from effective research models to their practical, real-world application is crowded with both potential and problems.
- Challenge 1: Data quality and consistency
- The research model is heavily reliant on the quality and chronological correctness of the data.
- Solution: For replication and real-world applications, particularly in low-resource settings, developing simplified, standardized data input tools or utilizing mobile applications that health workers can use to enter data in real-time, ensuring consistency and reliability.
- Challenge 2: Systematic variations in global health data
- Applications across different healthcare systems (for example, the DNPR to US-VA transition involves applying the AI model, originally developed using data from the Danish National Patient Registry (DNPR), to patient data from the US Veterans Affairs (US-VA) healthcare system.) noted a decrease in performance.
- Solution: The creation of a robust, flexible model adaptation guideline that can guide researchers and practitioners across different global regions to adopt the model with minimum information loss or misinterpretation, coupled with localized training sessions to ensure the model is accurately adapted.
This research has the potential to be a keystone in promoting global health equity by incorporating such precise, structured solutions and putting them into practice while carefully taking into account localized settings.
The entwined strands of AI technology and health equity create a reality that depicts the real prospects of changing the global health landscape, particularly in low-resource situations. In countries where late-stage detection is a stark reality due to a lack of infrastructure or expertise, the AI model presents a strong potential for early identification of pancreatic cancer. This can improve healthcare outcomes in those regions. Healthcare systems can better prioritize and allocate their resources by anticipating possible cancer occurrences. This will ensure that prompt interventions are made even when resources are limited. The technical and knowledge gap across various healthcare settings globally could be minimized by an open-source AI model adaptation that encourages international collaboration and information exchange.
This portrays the model not just as a tool for bettering healthcare outcomes but also as a beacon that shows the way toward a just and universal distribution of health resources. How can we further eliminate barriers within this framework to guarantee the seamless adoption and use of such AI models, strengthening the road to global health equity?
Source Article: https://www.nature.com/articles/s41591-023-02332-5#Abs1
Disclaimer: Please note that the opinions, content, and analysis in my posts are entirely my own and do not reflect the views of any current or past employers or institutional affiliations. These posts, based solely on publicly available information, are for informational purposes and should not be taken as professional advice. All insights and conclusions are my viewpoints and should not be considered representative of any organizations I am or have been associated with. This content is not endorsed by, nor does it represent the stance of any affiliated entity.





