
The word “Parkinsonism” is used to refer to a broad category of neurological conditions that have comparable motor symptoms. Tremors, slowing down of movement, and rigidity are some of these symptoms. Although the most well-known type of Parkinsonism is Parkinson’s disease (PD), not everyone exhibiting these symptoms has PD.
In the past, PD diagnosis has been challenging. Currently used methods for diagnosing Parkinson’s disease (PD) combine neurological exams, patient history reviews, and clinical assessments. Before making a diagnosis of Parkinson’s disease (PD), a neurologist would typically seek at least two of the three main motor symptoms: stiffness, delayed movement, and tremor. However, there are potential pitfalls associated with these methods:
- Subjectivity: A significant portion of the diagnosis relies on subjective evaluations by clinicians and self-reports by patients, which can introduce biases and inaccuracies.
- Absence of definitive tests: Unlike many other conditions, PD lacks a straightforward diagnostic test. Blood tests or brain scans can’t definitively pinpoint the disease, leading to potential misdiagnoses.
- Varying progression rates: The progression of PD symptoms can be inconsistent among patients, making early detection even more challenging. Given the progressive nature of the disease, early and accurate diagnosis is pivotal in managing symptoms and ensuring an improved quality of life.
The difficulties outlined above necessitate a more streamlined, impartial, and effective method of diagnosing Parkinson’s disease (PD), paving the way for possible technological interventions such as Artificial Intelligence (AI).
With its ability to recognize patterns and digest data, artificial intelligence presents a viable replacement for conventional methods of Parkinson’s disease diagnosis. This paper presents a novel approach to diagnosing Parkinson’s disease (PD) by using artificial intelligence (AI) to examine nighttime breathing patterns. According to the National Institute of Health, this could be a game changer because, early in the course of Parkinson’s disease, parts of the brain that govern respiration and sleep tend to be impacted. How is this operational?
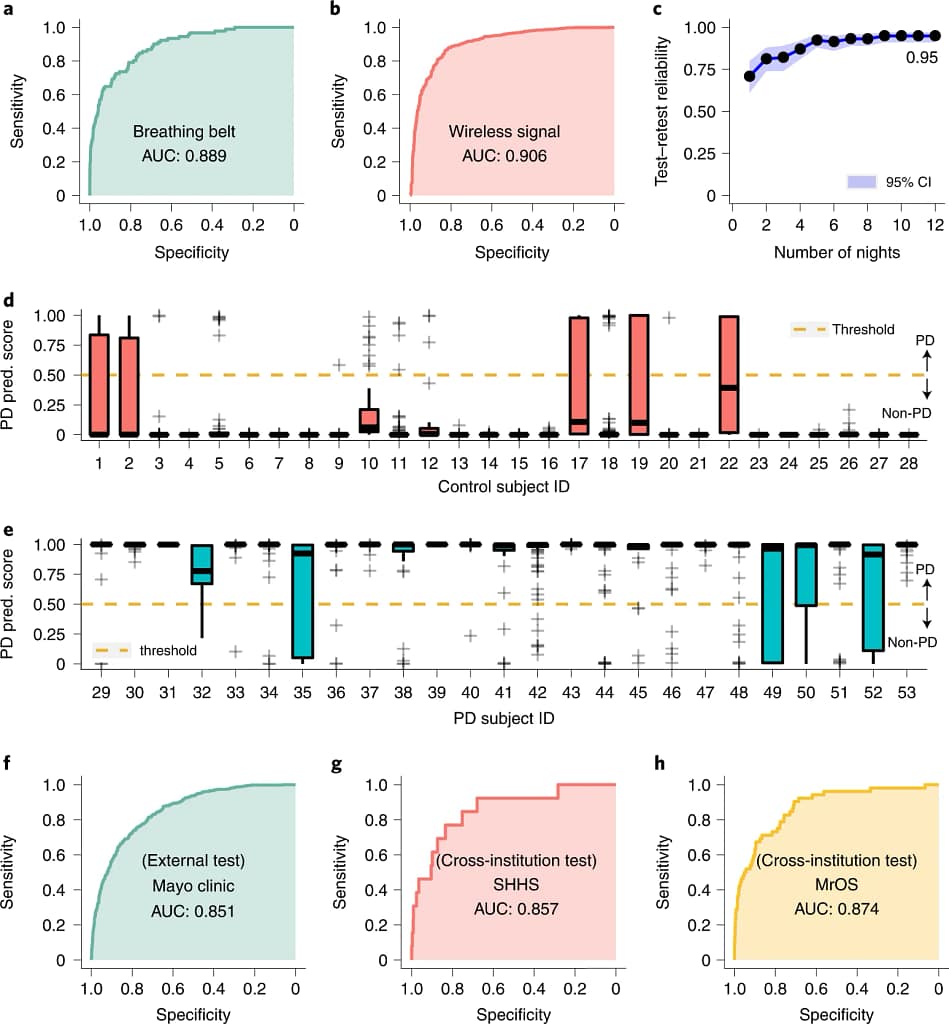
- Breathing belt data: Using a breathing belt, which measures chest and diaphragm movements during respiration, the AI model attained an area under the ROC curve (AUC) of 0.889, with sensitivity and specificity of 80.22% and 78.62%, respectively. The ROC curve, a graphical plot, represents the model’s ability to correctly classify those with and without PD. A higher AUC means the model is more effective at identifying genuine PD cases.
- Wireless signal data: With the advent of technology that captures breathing patterns via wireless signals, we can envision them as non-invasive, distant biometric sensors. When the AI model used this data source, it achieved an AUC of 0.906 along with an impressive sensitivity of 86.23% and specificity of 82.83% in PD detection, showcasing the model’s robustness across varied data streams.
- Significance of one-night data: The research underscored the innovation of diagnosing PD with just a single night’s nocturnal breathing data. This approach paves the way for non-intrusive diagnostic tools, making regular monitoring feasible without taxing the patient.
- Transfer learning’s role: An indispensable technological component in the AI model’s success. Transfer learning lets a model trained on one task adapt to a different, yet similar task. In the context of PD, it means harnessing insights from related respiratory disorders to fine-tune PD diagnosis.
For people exhibiting early non-motor signs of Parkinson’s disease (PD), such as mood disorders or sleep issues, the cutting-edge AI technology for PD diagnosis is extremely relevant. Most likely to benefit are individuals with a direct family history of Parkinson’s disease (PD), substantial prior head injuries, long-term exposure to chemicals such as solvents, herbicides, or pesticides, or those over 60. This method, which ensures early detection and intervention, is best suited for individuals with heightened risk factors or early symptoms rather than taking a general approach.
Transitioning from lab-controlled research settings to real-world settings presents significant difficulties, particularly in resource-poor areas. Let us analyze the possible obstacles that the research may encounter in modifying its AI model for various environments and present practical remedies that are customized to the particular circumstances of the study:
- Variability in data collection environments:
- Challenge: In a controlled environment, factors like ambient noise, device positioning, or even patient movement are kept minimal. Such consistency may not be possible in diverse home settings, potentially leading to data discrepancies.
- Solution: Enhance the AI’s preprocessing capabilities to filter out ambient disruptions and include an instructional module with the device to guide optimal setup and positioning.
- The broad spectrum of disease presentation:
- Challenge: Parkinson’s disease manifests with varied symptoms and severity across patients. The study’s data might have encompassed a limited range of these variations, and extrapolating it to diverse populations could lead to potential misdiagnoses.
- Solution: Collaborate with international research groups to gather and integrate diverse patient data, thereby refining the model to recognize a wider range of PD presentations.
- Limited access to technology in low-resource settings:
- Challenge: Not every community has access to regular wireless connectivity or even a continuous power supply, essential for the study’s AI-driven wireless monitoring.
- Solution: Develop a version of the device with local storage capabilities, allowing data collection without real-time connectivity. Couple this with solar charging options to ensure uninterrupted functioning.
- Inherent biases in AI training data:
- Challenge: The AI’s accuracy is contingent upon the data it’s trained on. If the initial data predominantly represents a certain demographic, the AI might exhibit biases, reducing its diagnostic efficacy in other populations.
- Solution: Actively source diverse datasets, especially from underrepresented regions, and periodically retrain the model to ensure comprehensive and unbiased PD diagnosis.
The use of this AI technology has enormous potential to promote equity in global health. According to a 2019 WHO estimate, 8.5 million individuals worldwide suffer from Parkinson’s disease, with Oman, Qatar, and the United Arab Emirates having the highest prevalence. This approach aims to give early and reliable diagnosis of Parkinson’s disease (PD) by tackling the issues of prevalence discrepancies, model generalization, complicated datasets, and remote monitoring. Regardless of their socio-economic status, PD patients’ quality of life can be greatly enhanced by enabling early interventions. This embodies the objective of global health equity: providing all people, wherever, with equal access to healthcare resources.
But even as we welcome this AI era with Parkinson’s diagnosis, one question still needs to be answered: Are we ready to adopt, use, and—above all—trust AI with our health in a world that is moving quickly toward digital health?
Source Article: https://www.nature.com/articles/s41591-022-01932-x#Fig2
Disclaimer: Please note that the opinions, content, and analysis in my posts are entirely my own and do not reflect the views of any current or past employers or institutional affiliations. These posts, based solely on publicly available information, are for informational purposes and should not be taken as professional advice. All insights and conclusions are my viewpoints and should not be considered representative of any organizations I am or have been associated with. This content is not endorsed by, nor does it represent the stance of any affiliated entity.





